Learn
Delve into dermatology diseases with new approaches from conventional to complementary care.
Topics
Explore a broad spectrum of dermatological conditions and topics to enhance your practice.
Training Programs
Expert led instruction for every level of patient care.
Log in to see enrolled program content.
Conferences
Gain practical insights and meet new colleagues.
Hover over a conference to view recordings.
Join Us
The largest integrative dermatology community.
Biosimilars: Where Are We Now?
Course Overview
Preview what you'll learn in this program
What You'll Learn
Biosimilar medications are "highly similar" to an already FDA-approved biological product. Several biosimilars have already been approved in the US for the treatment of psoriasis, with more sure to follow in the near future. The FDA is responsible for and has a process for ensuring the safety and efficacy of bio similars.
Learning Objectives
- Describe the scientific and developmental principles behind biosimilars.
- Analyze the available efficacy and safety/tolerability data.
- Identify the anticipated biosimilar approvals and considerations for use in clinical practice.
Faculty

Editors

Accreditation Information
Biosimilar medications are "highly similar" to an already FDA-approved biological product. Several biosimilars have already been approved in the US for the treatment of psoriasis, with more sure to follow in the near future. The FDA is responsible for and has a process for ensuring the safety and efficacy of bio similars.
Disclaimer
This website – LearnSkin by LearnHealth Inc. - and the information provided on the website is for educational purposes only. The information provided does not replace advice from a physician or a qualified health provider. The information provided on this website should never be used as a substitute for medical advice, diagnosis or treatment of health conditions or problems and should never be used to disregard, delay or refuse treatment. LearnHealth Inc. makes no warranties nor express or implied representations whatsoever regarding the accuracy, completeness, timeliness, comparative or controversial nature, or usefulness of any information contained or referenced on this Website. LearnHealth Inc. does not assume any risk whatsoever for your use of this Website or the information contained herein. Health-related information changes frequently and therefore information contained on this Website may be outdated, incomplete or incorrect. Statements made about products are not official statements made by the Food and Drug Administration. Use of this Website does not create an expressed or implied physician-patient relationship. You are hereby advised to consult with a physician or other professional health care provider prior to making any decisions, or undertaking any actions or not undertaking any actions related to any health care problem or issue you might have at any time, now or in the future. In using this Website, you agree that neither LearnHealth Inc. nor any other party is or will be liable or otherwise be responsible for any decision made or any action taken or any action not taken due to your use of any information presented.
✨ Ready to Begin Your Journey? ✨
Enroll now to start the course and unlock all learning materials
Biosimilars: Where Are We Now?
Complete the previous section to unlock this lesson
Biosimilars: Where Are We Now? Module
Complete the previous section to unlock this lesson
Course Discussion
Join the conversation with fellow learners
Also Recommended
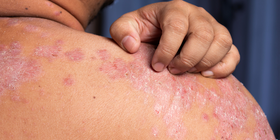
Integrative Approaches in Psoriasis: Combining Pharmacologic and Lifestyle Interventions
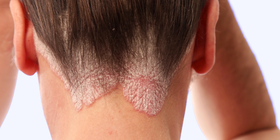
Psoriasis: Evolving Therapies and Whole-Patient Management

Extracutaneous Comorbidities of Psoriasis: Joint, Cardiovascular, Metabolic, and Psychological Impact


